In the rapidly evolving landscape of neurotechnology, Neuralink has emerged as a prominent and controversial player, pushing the boundaries of what’s possible in brain-computer interfaces (BCIs). Founded in 2016 by entrepreneur Elon Musk, Neuralink has captured public imagination with its ambitious goals and cutting-edge technology. This essay explores the significant contributions Neuralink has made to the field of BCIs and its potential impact on the future of human-computer interaction.
Revolutionary Implant Technology
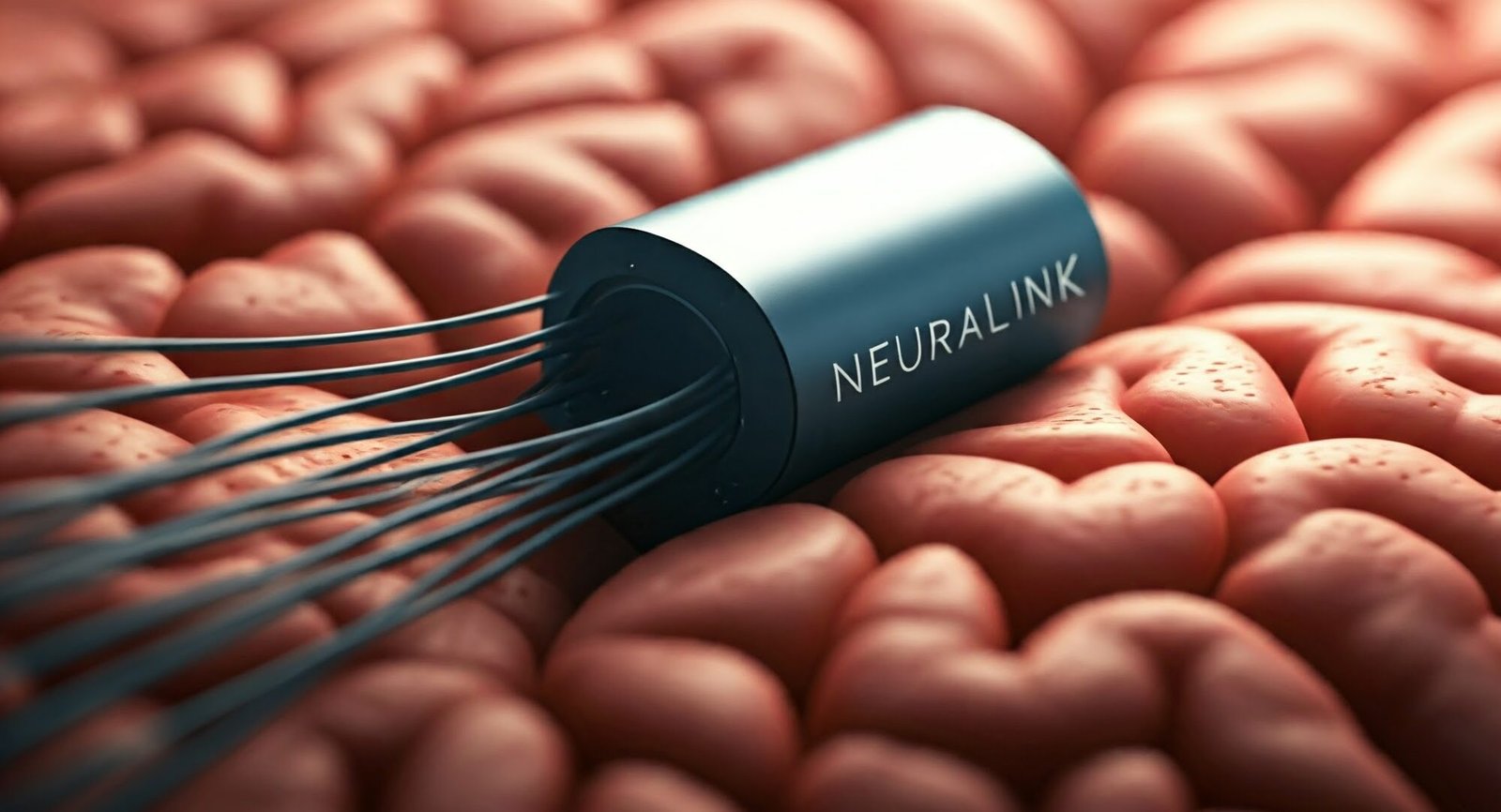
At the heart of Neuralink’s contributions is its innovative BCI implant, dubbed the “Link.” This device represents a significant leap forward in neural interface technology. Unlike traditional bulky implants, the Link is remarkably compact, about the size of a large coin. Its small form factor is not just a feat of miniaturization; it’s a crucial advancement that could make brain implants more feasible and less intrusive for human use.
The Link’s design incorporates ultra-thin, flexible threads that are finer than human hair. These threads can be precisely inserted into the brain to detect neural activity. This approach allows for higher resolution recording of brain signals compared to many existing BCIs, potentially offering more detailed and accurate brain-to-computer communication.
Enhancing Surgical Precision
Recognizing that the efficacy of a BCI is only as good as its implementation, Neuralink has developed a sophisticated robotic system for implanting its device. This robotic surgeon is designed to insert the thin neural threads with extreme precision, potentially reducing the risks associated with human error during implantation. By automating this delicate process, Neuralink aims to make the surgical procedure faster, safer, and less invasive – key factors in making BCI technology more accessible and appealing to potential users and medical professionals alike.
Wireless Capabilities and Data Processing
Neuralink’s device is designed to be fully implanted and wireless, a significant advancement over BCIs that require external components. This wireless capability could potentially reduce the risk of infection, a common concern with implanted medical devices that have external elements. Moreover, it offers users greater freedom of movement and could make the technology more seamless to use in everyday life.
To handle the vast amounts of data collected by the implant, Neuralink has invested in developing custom chips and sophisticated algorithms. This focus on data processing is crucial, as interpreting the complex signals from the brain quickly and accurately is essential for effective BCI operation.
Ambitious Vision and Public Engagement
While many BCI companies focus primarily on medical applications, such as helping paralyzed individuals regain movement, Neuralink has outlined more ambitious long-term goals. These include enhancing human cognition and enabling direct brain-to-computer communication. While these objectives remain largely theoretical, they have sparked intense public interest and debate about the future of human-computer interaction.
Through high-profile demonstrations and announcements, Neuralink has significantly raised public awareness about BCI technology. This increased visibility has not only generated excitement but also prompted important discussions about the ethical implications and societal impact of such technology.
Progress in Animal Trials
Neuralink has made significant strides in animal trials, demonstrating the potential of its technology. Notable experiments include demonstrations of a monkey playing video games and controlling a cursor using the Neuralink implant. These trials have provided valuable insights into the functionality and potential applications of their BCI system.
Advancing Towards Human Trials
In a major milestone, Neuralink received FDA approval in 2023 to begin human clinical trials. This approval marks a crucial step in moving the technology from theoretical and animal studies to practical applications in humans. It also subjects Neuralink’s technology to rigorous scientific scrutiny and regulatory oversight, which is essential for ensuring safety and efficacy.
Challenges and Future Prospects
Despite its promising advancements, Neuralink faces significant challenges. The long-term safety and efficacy of its technology remain to be proven through extensive clinical trials. Ethical concerns about brain augmentation and data privacy need to be addressed. Moreover, translating success in animal trials to practical, beneficial applications in humans is a complex process that will require time, further research, and possibly technological refinements.
Neuralink’s contributions to the field of brain-computer interfaces have been substantial and multifaceted. From innovative hardware design to advanced surgical techniques, from ambitious goals to public engagement, the company has pushed the boundaries of what’s possible in BCI technology. While many of its proposed applications remain to be realized, Neuralink has undoubtedly accelerated progress in the field and sparked crucial discussions about the future of human-computer interaction.
As Neuralink moves forward with human trials and continues its research, it has the potential to revolutionize not just how we interact with computers, but how we understand and augment human cognition itself. However, this journey will require careful navigation of scientific, ethical, and regulatory challenges. The coming years will be crucial in determining whether Neuralink’s ambitious vision can translate into practical, safe, and beneficial technologies that truly enhance human capabilities and improve lives.
To provide a more comprehensive understanding of Neuralink’s work and its impact, here are some key resources:
Official Sources
- Neuralink’s official website: https://neuralink.com/
This is the primary source for official information about the company’s technology and mission. - Neuralink’s YouTube channel: https://www.youtube.com/@neuralink
Features demonstrations and explanations of their technology.
Scientific Publications
- Musk, E., Neuralink (2019). “An integrated brain-machine interface platform with thousands of channels.” Journal of Medical Internet Research, 21(10), e16194.
https://www.jmir.org/2019/10/e16194/
This paper provides technical details about Neuralink’s BCI system.
News Articles and Analysis
- “Elon Musk’s Neuralink Gets FDA Approval for Human Clinical Study” – The Wall Street Journal, May 25, 2023
https://www.wsj.com/articles/elon-musks-neuralink-gets-fda-approval-for-human-clinical-study-e5b78f36 - “What Is Neuralink? We Explain Elon Musk’s Brain Chip Company” – Time, December 3, 2022
https://time.com/6238625/neuralink-elon-musk-explained/
Critical Perspectives
“Neuralink: The hype and the reality” – Nature, September 1, 2023
https://www.nature.com/articles/d41586-023-02748-6
Offers a balanced view of Neuralink’s claims and the scientific reality.
These resources provide a mix of official information, scientific literature, news coverage, and critical analysis. They offer a well-rounded view of Neuralink’s contributions, progress, and the discussions surrounding its technology. When reviewing these sources, it’s important to consider the date of publication, as the field of BCIs and Neuralink’s work are rapidly evolving.